Binding, or adsorbing, specific mycotoxins to limit their negative effects in livestock is a well-established method for mycotoxin deactivation. While a large number of binder products containing clay minerals such as bentonites are commercially available, there is a certain amount of confusion in the market regarding claims authorized by the European Commission.
EXPERT
This matters to many feed and livestock producers, since it relates to product safety and effectiveness which in turn impacts animal performance and profitably.
What can be bound?
This can be answered on 2 levels:
Starting from the chemistry, mycotoxins such as aflatoxins have a flat chemical structure and can be trapped between the layers of bentonites, in the same way a slice of meat sits between 2 slices of bread in a sandwich. Once the mycotoxin enters the binder layers, the electric force generated by the atoms of both compounds tightens the bond. The less flat chemical structure of other mycotoxins like deoxynivalenol (DON) or zearalenone (ZEN) results in less effective adsorption. Legally speaking, only aflatoxin binding claims are authorised in the EU.
What makes a good binder?
A multi-year research project between Biomin and IFA Tulln, the world leader in research on fungi and mycotoxins, tested more than 300 different materials e.g. organic binders, cellular components, aluminosilicates, activated carbon, etc. for their ability to bind aflatoxins. 5 key characteristics defined a successful material, namely:
- High adsorption capacity
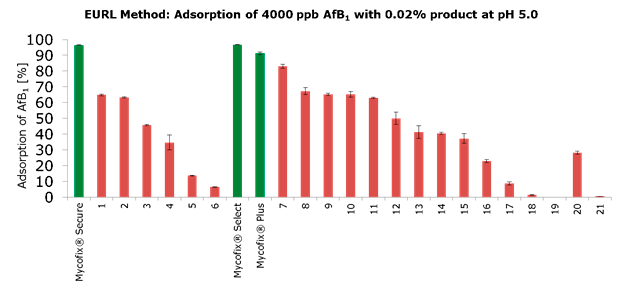
The method developed by IFA and the Biomin Research Center requires that 200 grams of the adsorbent be able to bind more than 90% of 4,000 ppb of aflatoxin at a pH of 5.0. This is a rather high bar, as the chart below shows that only 3 out of 30 commercially-available products tested met the requirements. This set of requirements was later adopted as a reference for testing adsorbent materials by the European Union Reference Laboratories (EURL).
- Irreversibility
It is important that the aflatoxin binding is strong e.g. not easily undone – otherwise the bound toxins could be released again and impair animal performance. - Specificity
Specificity means that only the targeted material (aflatoxins) is adsorbed. A material that is not specific would bind all sorts of other things, such as nutrients, and reduce the quality of feed – a particularly undesirable effect when it comes to feed additives. - Safety
Any binder used in the food and feed chain should by definition be safe for animals, consumers and the environment. In practice, this means that the substance should be non-toxic and have no carry-over into meat and other animal products. - In vivo biomarkers studies
Data from a minimum of 3 in vivo studies performed in at least 2 different locations showing statistically significant effects must be provided to demonstrate efficacy at the lowest recommended dosage in a specific species. Demonstration of efficacy must be provided according to scientifically recognised biomarkers for target species.
And the winner is…
These 5 criteria are reflected in the EU authorisation process that governs claims of mycotoxin deactivation. The Biomin – IFA project allowed researchers to identify a particular bentonite for its outstanding aflatoxin binding abilities. This bentonite was scientifically evaluated by EFSA and obtained the EU authorisation for mycotoxin deactivation – a testament to its safety, efficacy and purity.
EU authorisation
In 2009, the European Commission opened a new functional group of technological additives in order to ensure safety, purity and efficacy of mycotoxin deactivating compounds. EU registration is considered a benchmark for quality by the industry and markets, also outside the EU. Feed and livestock producers are able to make decisions more wisely knowing that they are purchasing quality products.
In total, 2 official documents report the necessary requirements to attain registration:
The stringent EU requirements to directly prove the deactivation of mycotoxins in vivo with scientific biomarkers demands a solid and long-standing commitment to R&D in the service of customers. It involves a significant amount of in vivo and in vitro data. So far, only one company (Biomin) has achieved EU authorisation for substances able to detoxify mycotoxins: one authorisation for the bentonite (Mycofix® Secure) plus 2 other substances (FUMzyme® and Biomin® BBSH).
Apart from Biomin, 5 other companies tried to receive EU authorisation for their product for aflatoxin-binding, but none of them succeeded so far and 4 of them have already withdrawn their dossiers.
Misguiding claims
In some cases, companies that produce binders will claim to have included some proportion of bentonite or have EU authorisation for their products without having had the product evaluated for safety and efficacy. This warrants caution by feed and livestock producers, as the product on offer may not contain the correct bentonite or the appropriate amounts in order to be effective. Moreover, those binder providers expose themselves to investigation of the claims by national control authorities, which can demand the evidence of scientific backing. Bentonite is a natural clay and differs largely depending on the origin. Only the specific bentonite sold exclusively in the Mycofix® product line has undergone the complete EFSA evaluation with all experiments and trials for identity, safety and efficacy and succeeded in a final authorisation.
Buyer beware
The EU authorisation as “substances for reduction of the contamination of feed by mycotoxins” it is a must in terms of quality for feed and livestock producers. In the case of the bentonite 1m558 as aflatoxin binder, this authorisation is based on the dossier submitted by Biomin and on the bentonite available from the company. We at Biomin have proven that our bentonite works with an extensive dossier positively evaluated by EFSA and meeting all the requirements of efficacy, selectivity and safety. Potential pitfalls of a bentonite lacking this scientific evaluation could include poor efficacy, reduction in feed quality, concerns around safety and wasted money. Given the highly competitive nature of today’s global animal protein markets, robust scientific data and proper authorisation can offer both performance (in terms of efficacy) and peace of mind.

Ignacio
Ignacio holds a bachelor in Agriculture Sciences and a masters in Sustainable Animal Nutrition and Feeding. He´s the global product manager within the Mycotoxin Risk Management team of DSM-Firmenich, with experience in supporting farmers, nutritionists and health experts to overcome the negative impact of mycotoxins in different parts of the World.